Finished Device Assembly and Packaging
Finished Medical Devices for MedTech and Pharma Customers
Cadence prides itself on a strong track record of regulatory compliance. All our facilities are ISO 13485 certified and registered with the FDA where applicable. Our robust Quality Management System (QMS) ensures compliance with all regulatory requirements and standards.
We provide the following finished device capabilities:
- Cleanroom Manufacturing (Class 7 and Class 8)
- Final Assembly
- Testing and Inspection Services
- Final Packaging and Labeling
- Sterilization Management
- Supply Chain Management
Final Assembly of Medical Devices
The final assembly of medical devices is completed in tightly controlled environments with precise processes to ensure the proper function of the device while meeting regulatory requirements. These products often get assembled, packaged, labeled, sterilized, and are shipped directly to our customers’ manufacturing and distribution facilities. The integrity of the device is paramount as it impacts patient safety and is a critical step in the medical device life cycle.
Cadence operates two dedicated finished device assembly facilities in Cranberry Township, PA and Alajuela, Costa Rica. Both facilities maintain ISO Class 7 cleanrooms, are ISO 13485 certified, and assemble, package and ship Class I and Class II FDA registered products. We partner with our customers through the many stages of the product lifecycle, from engineering and operations support during the pre-clinical phases, to new production introduction, through product transfers to improve supply-base scalability or to take advantage of nearshore costs. Our experienced engineering, quality, supply-chain and operations teams are ready to help across the many stages of your product development process.
Some of our medical device qualifications include:
- Class 7 Certified Cleanroom
- Class 8 Certified Cleanroom
- Cleanroom Injection and Insert Molding
- cGMP / CFR 21 Part 820 Compliant
- FDA Registered Products/Facilities
- ISO 13485:2016 certified
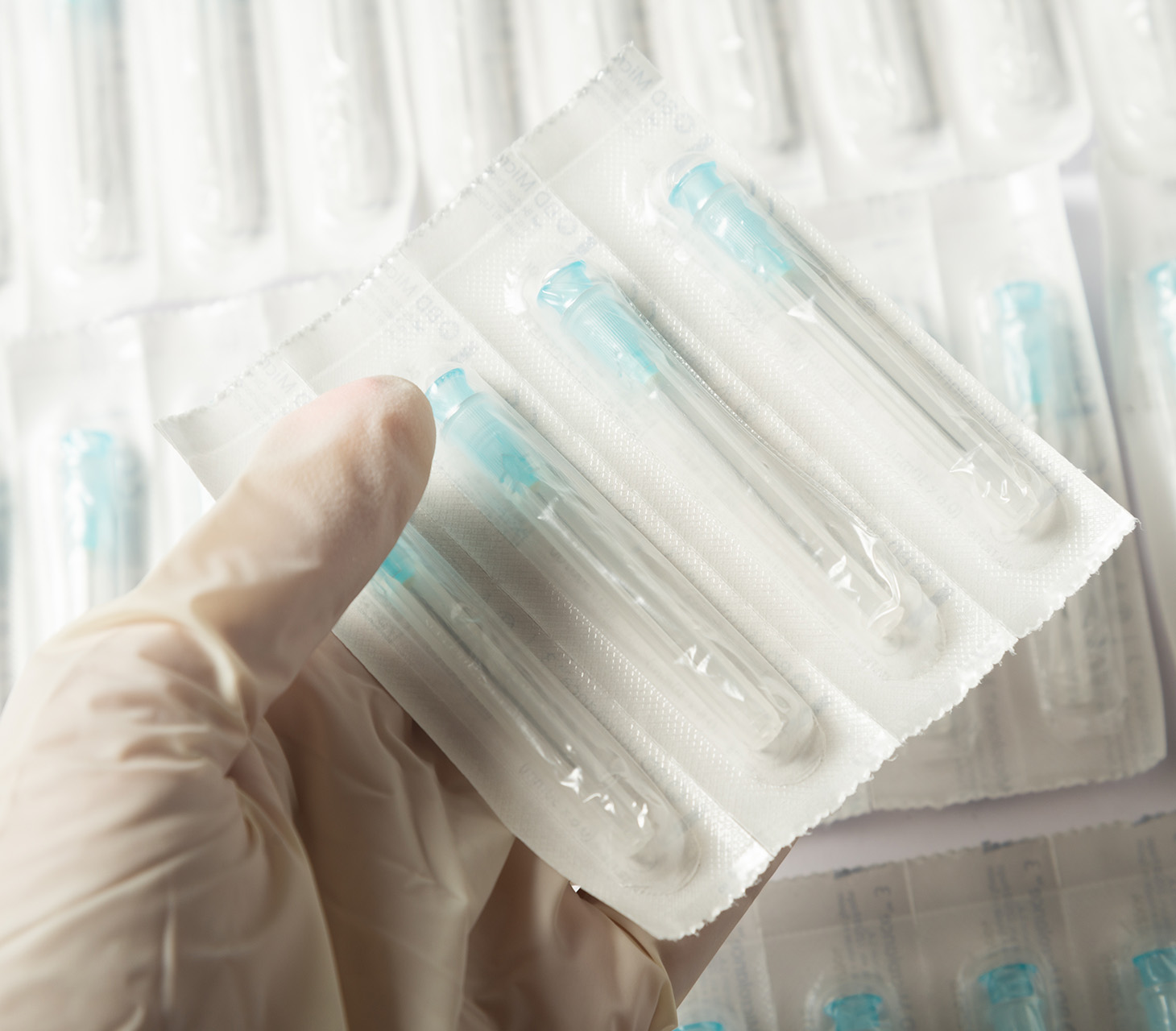
Final Packaging & Labeling for Medical Devices
Our packaging expertise lies in design support, development and validation of custom packaging of precision components, sub-assemblies and finished medical devices including related processes in compliance with the appropriate regulatory standards. This includes understanding complex needs for pouching, labeling, sealing, shrink-wrapping, and boxing.
Cadence’s expertise in “sharps” translates directly to the handling throughout the entire manufacturing process, including through semi-automated and fully automated assembly solicitors. Our engineering team provides support through the entire development process to minimize damage to highly engineered precision products.
How Can We Help You?
"*" indicates required fields
* indicates required fields
